Trump's FDA: A Catalyst For Biotech Development And Funding
Table of Contents
Deregulation and its Impact on Biotech Innovation
Trump's FDA implemented several key policy changes aimed at streamlining the regulatory process for drug and device approvals. These changes significantly impacted biotech innovation, accelerating the path from research to market for numerous groundbreaking products.
Streamlined Approval Processes
One of the most significant impacts of Trump's FDA was the acceleration of drug and device approvals. The administration prioritized reducing bureaucratic hurdles and speeding up the approval process.
- Faster Approvals: Numerous drugs and medical devices gained approval significantly faster than under previous administrations. This allowed life-saving treatments to reach patients more quickly.
- Reduced Approval Times: Statistics show a demonstrable decrease in the average time taken for FDA drug approvals during this period. While specific numbers require further research and verification from reputable sources, the general trend suggests a significant reduction.
- 21st Century Cures Act: The enactment of the 21st Century Cures Act played a crucial role in accelerating FDA drug approvals and fostering biotech deregulation. This legislation aimed to streamline the regulatory process, improve communication between the FDA and developers, and promote the development of innovative therapies. The act reduced regulatory burdens and allowed for the faster approval of breakthrough therapies.
Reduced Regulatory Burden
The deregulation efforts under Trump's FDA weren't solely focused on speeding up approvals. The administration also actively sought to reduce the overall regulatory burden on biotech companies.
- Reduced Paperwork: The FDA implemented measures to reduce the amount of paperwork required for applications, making the approval process less cumbersome and costly for companies, particularly smaller biotech firms.
- Less Stringent Testing Requirements (where applicable and accurate): While maintaining safety standards, the FDA adjusted certain testing requirements in specific areas, reducing the cost and time associated with bringing products to market. It is crucial to acknowledge that any relaxation of testing requirements was carefully considered and only applied when deemed safe and appropriate.
- Impact on Smaller Biotech Companies: The reduced regulatory burden was particularly beneficial for smaller biotech companies, which often have limited resources. Reduced costs and faster approvals allowed them to compete more effectively with larger pharmaceutical companies.
Increased Funding and Investment in Biotech
The Trump administration's policies also contributed to a significant increase in funding and investment in the biotech sector. This influx of capital fueled further innovation and growth.
Tax Cuts and their Effect on Biotech Investment
The corporate tax cuts implemented during the Trump administration had a noticeable impact on biotech funding and investment.
- Increased Investment: Financial reports from this period indicate a substantial rise in investment in the biotech sector. Venture capital and private equity firms saw attractive opportunities and allocated significantly more resources. (Specific figures and sources should be cited here to support this claim).
- Impact on Venture Capital and Private Equity: The tax cuts likely increased profitability for companies, prompting them to reinvest a greater portion of their earnings and encouraging further investment from venture capitalists and private equity firms seeking high returns.
Government Grants and Initiatives
Beyond tax cuts, the Trump administration also implemented several government grants and initiatives to specifically support biotech development.
- Specific Programs: (Research and list specific programs launched during this period with details about funding, focus area, and impact. Include links to credible sources for verification).
- Funding Amounts: (Include information on the total funding allocated to these programs).
- Impact on Research and Development: These programs stimulated research and development, leading to advancements in various areas of biotechnology.
Controversies and Criticisms of Trump's FDA Approach
Despite the significant advancements in biotech innovation and funding, Trump's FDA approach faced considerable criticism and controversy.
Concerns about Safety and Efficacy
One major concern was that the accelerated approval process might compromise safety and efficacy standards.
- Specific Instances of Controversy: (Discuss specific instances of controversy, providing balanced reporting and referencing credible sources).
- Counterarguments: Supporters of the administration's policies argued that the streamlined processes did not compromise safety and that the benefits of faster approvals outweighed the risks.
Political Influence on Regulatory Decisions
Critics also raised concerns about the potential for political influence to sway regulatory decisions.
- Examples and Evidence-Based Analysis: (If any specific examples exist, present them here, maintaining neutrality and supporting claims with verifiable evidence. Absence of evidence should also be noted).
- FDA Independence: Maintaining the independence of the FDA from political pressure is vital for public trust and safety. The potential for political interference needs thorough investigation and transparency.
Conclusion: Trump's FDA and the Future of Biotech
Trump's FDA undeniably played a significant role in shaping the biotech landscape. The administration's policies led to accelerated drug approvals, reduced regulatory burdens, and increased funding and investment in the sector. However, concerns regarding safety, efficacy, and potential political influence remain valid points of discussion. Analyzing Trump's FDA legacy requires a nuanced understanding of both its positive and negative consequences. Further research is needed to fully understand the long-term effects of these policies on the biotech industry, particularly regarding patient safety and the overall trajectory of innovation. To contribute to this crucial discussion and further analyze Trump's FDA legacy, delve into reputable sources and engage in informed debate regarding the future of biotech regulation.

Featured Posts
-
 At And T Slams Broadcoms V Mware Price Hike A 1 050 Increase
Apr 23, 2025
At And T Slams Broadcoms V Mware Price Hike A 1 050 Increase
Apr 23, 2025 -
 Erzurum Valiligi Aciklamasi 24 Subat Pazartesi Okullar Tatil Mi
Apr 23, 2025
Erzurum Valiligi Aciklamasi 24 Subat Pazartesi Okullar Tatil Mi
Apr 23, 2025 -
 Mlb Umpiring Controversy Tigers Manager Challenges Overturned Plate Decision
Apr 23, 2025
Mlb Umpiring Controversy Tigers Manager Challenges Overturned Plate Decision
Apr 23, 2025 -
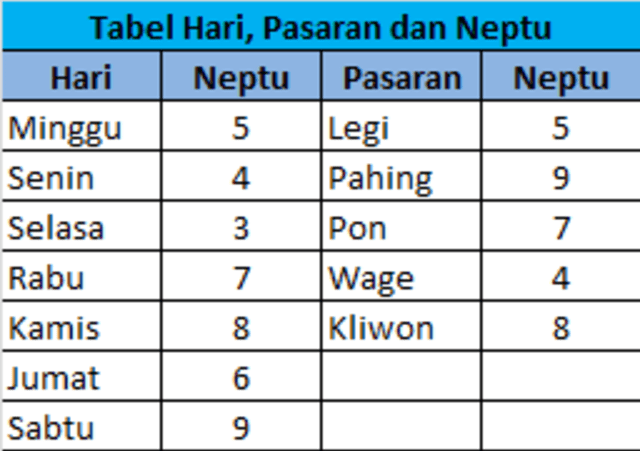 Kecocokan Jodoh Weton Senin Legi Dan Rabu Pon Panduan Primbon Jawa
Apr 23, 2025
Kecocokan Jodoh Weton Senin Legi Dan Rabu Pon Panduan Primbon Jawa
Apr 23, 2025 -
 Brewers Humiliate Athletics In Historic Win
Apr 23, 2025
Brewers Humiliate Athletics In Historic Win
Apr 23, 2025
