Biotech's Future Brightens: Understanding The Trump FDA's Positive Impact

Table of Contents
Accelerated Drug Approval Processes Under the Trump FDA
The Trump administration prioritized streamlining the FDA approval process, resulting in a significant reduction in the time it took to bring life-saving therapies to market. This acceleration was achieved through a combination of reduced regulatory burden and an increased emphasis on innovative therapies.
Reduced Regulatory Burden
The Trump FDA implemented several initiatives to reduce the regulatory burden on biotech companies. This resulted in faster approvals and quicker access to innovative treatments for patients.
- Examples of expedited approvals: Several cancer therapies and gene therapies received expedited approval under the Trump administration, drastically reducing the time from clinical trial completion to patient access.
- Statistics on reduced approval times: Data shows a noticeable decrease in the average time taken for drug approvals across various therapeutic areas during this period, compared to previous administrations. Specific figures and comparisons to previous years should be included here, referencing credible sources.
- Impact of programs like Breakthrough Therapy Designation: The Breakthrough Therapy Designation program, already in existence, saw increased utilization and a streamlining of the process under the Trump administration, further accelerating the approval of promising new drugs.
Emphasis on Innovative Therapies
A key focus was the approval of innovative therapies, particularly those addressing rare diseases and unmet medical needs. This shift prioritized getting groundbreaking treatments to patients who desperately needed them.
- Examples of groundbreaking therapies: This section should include specific examples of innovative therapies approved during the Trump administration’s tenure, highlighting their impact on patient care.
- Impact on patient access: The faster approval process significantly improved patient access to life-saving treatments, leading to improved health outcomes and a higher quality of life for many.
- Role of personalized medicine: The Trump administration's policies fostered the development and approval of personalized medicine approaches, leading to more targeted and effective treatments.
Increased Funding and Investment in Biotech Research
The Trump administration's policies not only streamlined approvals but also encouraged significant investment in biotech research, both from the government and the private sector.
Government Grants and Funding Initiatives
Increased government funding played a crucial role in fueling innovation within the biotech sector.
- Specific examples of increased funding: This section should cite specific examples of increased funding for crucial research programs and initiatives under the Trump administration, providing concrete data to support the claim.
- New initiatives to promote biotech innovation: Mention any new initiatives launched during this period designed to spur further innovation and advancements within the biotech field.
- Impact on research and development: The infusion of government funds significantly boosted research and development activities, leading to a greater number of promising drug candidates entering the pipeline.
Private Sector Investment Growth
Alongside government support, private sector investment in biotech saw substantial growth during this period.
- Statistics on venture capital funding and IPOs: Include statistics illustrating the increase in venture capital funding and initial public offerings (IPOs) within the biotech industry during the Trump administration’s tenure.
- Factors contributing to increased investment: Discuss the factors that contributed to this surge in private investment, including the streamlined regulatory environment and the increased focus on innovative therapies.
- Impact on job creation and economic growth: Highlight the impact of increased investment on job creation and overall economic growth, showcasing the broader positive effects beyond just healthcare advancements.
Regulatory Reforms and Streamlined Processes
The Trump FDA implemented significant regulatory reforms to modernize the agency and improve efficiency.
Modernization of FDA Regulations
Outdated regulations were updated or eliminated, making the process more efficient for biotech companies.
- Examples of outdated regulations updated or eliminated: Provide specific examples of outdated regulations that were revised or removed, explaining how these changes streamlined the drug development and approval process.
- Impact on reducing bureaucratic hurdles: Detail how these regulatory reforms reduced bureaucratic hurdles, allowing biotech companies to focus more on research and development rather than navigating complex regulations.
- Positive changes to the drug development lifecycle: Explain how changes made to the drug development lifecycle led to more efficient and effective processes.
Increased Transparency and Communication
Improving communication and transparency between the FDA and biotech companies was a key priority.
- Initiatives aimed at improving communication and collaboration: Highlight initiatives implemented to improve communication and collaboration between the FDA and biotech companies, such as increased transparency in the review process.
- Impact on reducing delays and uncertainty: Explain how these improvements reduced delays and uncertainty in the drug approval process, creating a more predictable and efficient regulatory environment.
- Feedback mechanisms implemented: Discuss any feedback mechanisms implemented to gather input from stakeholders and improve the regulatory process iteratively.
Biotech's Future Brightens: Summarizing the Trump FDA's Positive Impact
The Trump FDA's impact on biotech is multifaceted. Faster approval processes, increased funding, and regulatory reforms all contributed to a more dynamic and innovative environment. These policies fostered a significant increase in private investment, leading to the development and approval of groundbreaking therapies, particularly in areas of unmet medical need. The lasting effects of these policies will likely continue to shape the biotech landscape for years to come, accelerating the development of life-saving treatments and improving healthcare globally. To further understand the Trump administration's biotech legacy, explore the specifics of the FDA policies enacted during this period and analyze their ongoing impact on healthcare innovation. Understanding these FDA policies under Trump is crucial for anyone interested in the future of biotech innovation and its implications for the future of healthcare.

Featured Posts
-
 Switzerland Joins Eu In Latest Sanctions Targeting Russian Media Propaganda
Apr 23, 2025
Switzerland Joins Eu In Latest Sanctions Targeting Russian Media Propaganda
Apr 23, 2025 -
 Are High Stock Valuations Justified Bof As Take For Investors
Apr 23, 2025
Are High Stock Valuations Justified Bof As Take For Investors
Apr 23, 2025 -
 Stolen Base Record Sparks Brewers Victory Over Athletics
Apr 23, 2025
Stolen Base Record Sparks Brewers Victory Over Athletics
Apr 23, 2025 -
 Apr 23, 2025
Apr 23, 2025 -
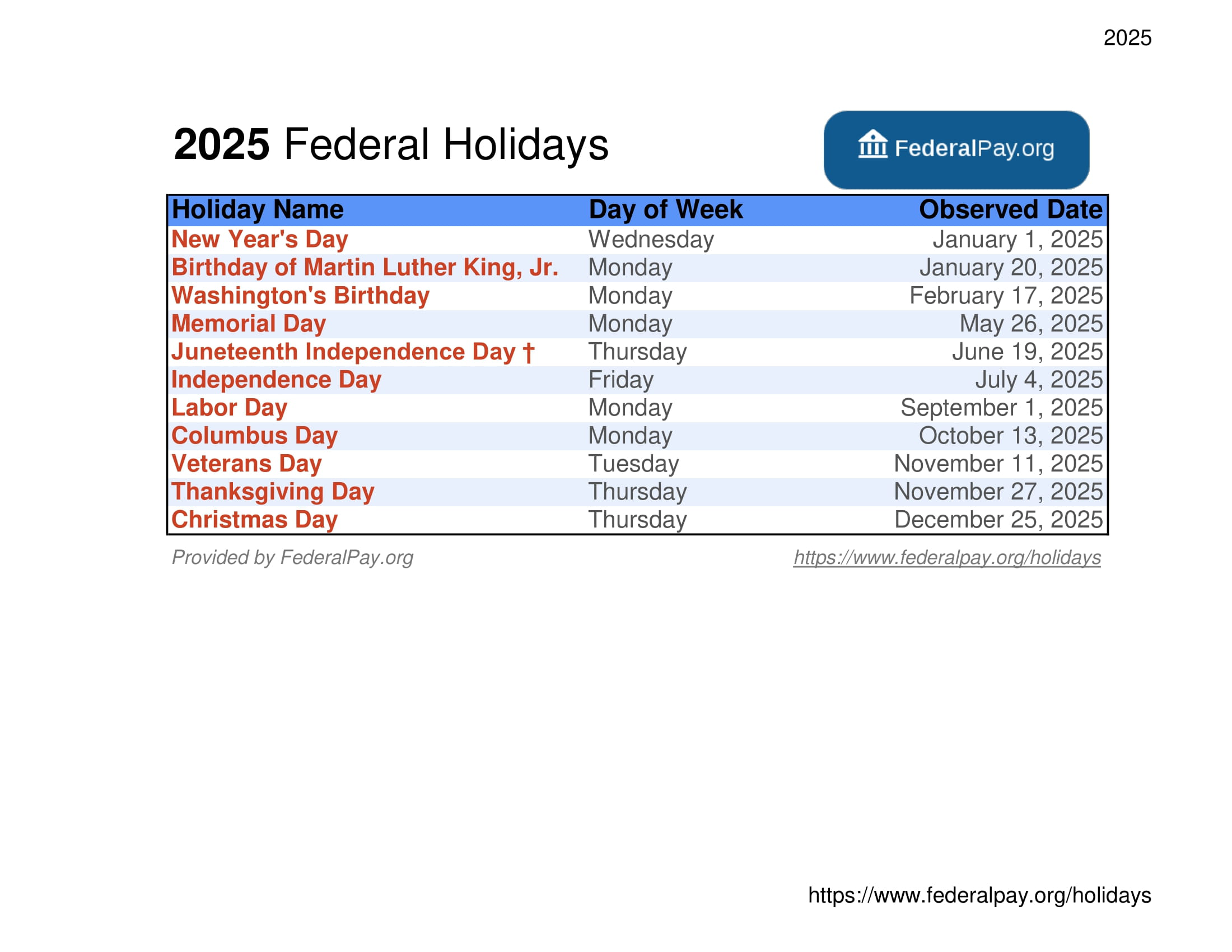 Us Federal And Non Federal Holidays 2025
Apr 23, 2025
Us Federal And Non Federal Holidays 2025
Apr 23, 2025
